
Is H2 Chemistry for you? Should you take this course if you struggled with chemistry in secondary school? You're not alone if you’re unsure whether to take this subject. Between its reputation for being one of the toughest H2 modules and the recent changes to the syllabus, it’s understandable why you might hesitate to choose it over other options.
Not to worry – here at Zenith, one of Singapore’s top JC tuition centres, we believe in empowering students with the knowledge they need to make an informed choice.
In this article, we’ll walk you through the latest curriculum changes, explore potential academic and career routes if you take H2 Chemistry, and demystify the core concepts that you’ll be learning.
What is H2 Chemistry?
A-Level H2 Chemistry is structured around 3 Core Ideas and 4 Extension Topics. The Core Ideas constitute the backbone concepts of chemistry as a whole, while the Extension Topics build off these fundamental principles and discuss the subject matter in more detail.
Your JC Chemistry journey will start with Matter, Structure and Properties, and Transformation. The concepts and theories you learn here will be applicable in the later chapters, namely, Chemistry of Aqueous Solutions, Organic Chemistry, Electrochemistry and Chemistry of Transition Elements.
While there’s no official data on the number of students taking H2 Chemistry, science stream students take it as one of the most popular subjects. It broadens your university prospects and opens up new career pathways, arming you with bread-and-butter concepts to kickstart your future.
What’s new in the 2026 H2 Chemistry syllabus?
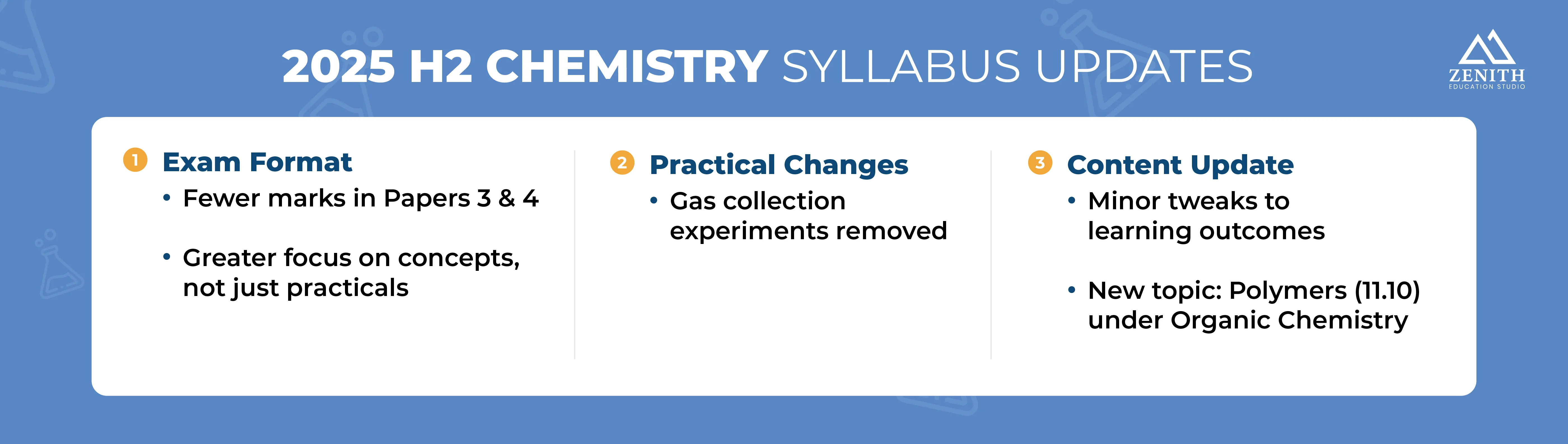
As one of the top tuition centres in Singapore, we’ve kept up with the latest updates to the H2 Chemistry syllabus. While there haven’t been any significant changes, minor adjustments to the exam structure, practical assessments, and subject content have been made. Here are some of the notable changes:
- There is a slight reduction in total marks from Papers 3 and 4, shifting the focus to strengthening conceptual understanding instead of hands-on application.
- Students will also no longer be required to carry out gas collection experiments.
- Minor changes to the learning outcomes of specific topics have been made, with the most significant change being the addition of Polymers (Topic 11.10) under Organic Chemistry.
These updates indicate that Syllabus 9729 places more emphasis on Knowledge and Understanding than Practical Application. Therefore, you must shift your approach to the exams by focusing on fully comprehending the core concepts.
Who should consider taking H2 Chemistry?
Taking H2 Chemistry is crucial for students who wish to work and pursue further studies in medicine, engineering, and related fields.
Often studied alongside other science and math modules, it is a common pre-requisite subject for science—and health-related degrees. So, if you have a specific dream course in mind, do find out which subjects are required for admission.
Here are some careers where chemistry knowledge will be essential:
- Chemist
- Doctor
- Chemical engineer
- Biomedical engineer
- Aerospace engineer
- Registered nurse
- Surgeon
- Pharmacist
- Forensic scientist
- Food scientist
- Soil and plant scientist
Why take H2 Chemistry?
Beyond unlocking academic and career pathways, the rigour of studying H2 Chemistry will encourage the development of many essential soft skills, including:
- Analytical and critical thinking
- Problem-solving skills
- Attention to detail
- Numeracy
- Natural curiosity
- Discipline and concentration
- Reasoning
- Scientific ethics
While it might be surprising that a single subject can cultivate many transferable skills, it’s important to remember that chemistry is a central science. Intersecting with other subjects such as math, biology and physics, the possible combinations sharpen your ability to approach complex problems methodically and draw connections between different disciplines.
What university courses require H2 Chemistry?
Here is a short list of top Singaporean university courses where H2 Chemistry is a pre-requisite subject:
How relevant would H2 Chemistry be in the future?
At the speed at which AI learning models continue to evolve, the fear of jobs being rendered obsolete in 2025 is a very real and concerning possibility.
According to data collected from job boards5, the jobs most at risk of being replaced are repetitive and require high accuracy. In contrast, jobs that work in tandem with AI or require human input and collaboration, such as those of a doctor or a chemical engineer, are least likely to be replaced.
Taking H2 Chemistry during your college years not only opens up career paths with upward growth predictions in the next decade, but it also positions fresh graduates at the helm of cutting-edge advancements with the aid of AI.

A-Level H2 Chemistry: Syllabus Outline
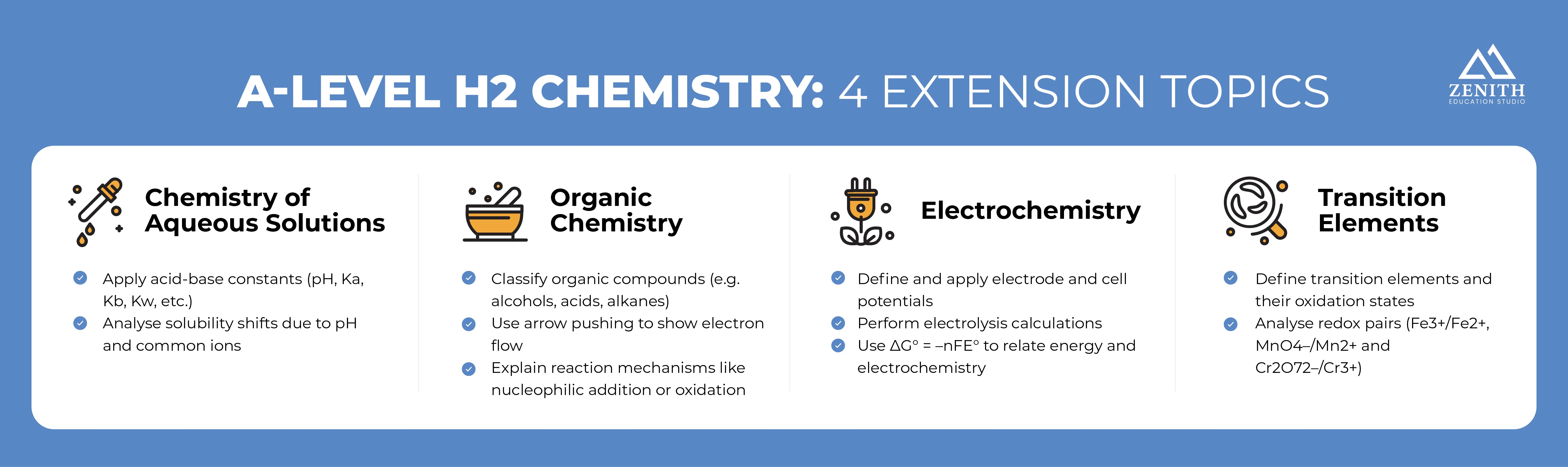
To put things simply, H2 Chemistry is about studying matter, its interactions, and transformations. Syllabus 9729 can be neatly categorised into two levels: 3 Core Ideas and 4 Extension Topics.
Core Ideas
Matter
The study of matter goes beyond pure academic interest—it forms the basic building blocks of life and influences our daily activities, from cooking to predicting weather patterns.
A good grasp of the atomic structure is essential to understanding the rest of the syllabus. By mastering concepts like ionisation energy, electron configurations, and complex ion formation, you will be better equipped to tackle more advanced topics later on, such as thermodynamics and electrochemistry.
Some of the learning outcomes include:
- Understanding the nucleus: identify and describe neutrons, protons and electrons; define isotopes, and deduce proton and nucleon numbers
- Deduce the behaviours of electrons: describe the shapes and relative energies of atomic orbitals, and explain the factors influencing ionisation energies
Structure and Properties
If you’ve ever wondered how airbags in cars can inflate within milliseconds or why biodegradable plastics can break down where other plastics can’t, this chapter will sate your curiosity.
In Structure and Properties, we build on our knowledge of atoms by exploring how different molecular arrangements can affect the substance’s behaviours and interactions. This core Idea covers several subtopics, including Chemical Bonding, the Gaseous State, Theories of Acids and Bases, and the periodic table.
Some of the learning outcomes include:
- Understanding chemical bonds: distinguish between ionic, covalent and metallic bonds
- Applying the general gas equation: use pV = nRT to determine Mr
- Master the theories of acids and bases: apply the Arrhenius, Brønsted-Lowry and Lewis theories
- Interpreting periodic trends: predict elemental properties and chemical systems based on their periodic table positioning
Transformation
Whether it’s maximising yield in an industrial process or using an instant cold pack to cool an injury, chemical transformations have been baked into the fundamentals of chemistry.
Next, we’ll introduce Transformations as the last Core Idea—a chapter that analyses how substances change and react, both qualitatively and quantitatively. The subtopics covered here include the Molecule Concept and Stoichiometry, Chemical Energetics: Thermochemistry and Thermodynamics, Reaction Kinetics, and Chemical Equilibrium.
Some of the learning outcomes include:
- Applying chemical formulae: calculate empirical and molecular formulae from combustion data
- Understanding enthalpy and entropy: predict thermodynamic feasibility through the concepts of Gibbs Free Energy, bond energy and lattice energy
- Explaining reaction energetics: define activation energy and describe the effect of catalysts using the Boltzmann distribution
- Define chemical equilibria: explain and relate reversible reactions and dynamic equilibrium
_V1.webp)
Extension Topics
Chemistry of Aqueous Solutions
Water-based solutions are present everywhere, from toothpaste to detergents and even down to the Blood Buffer System in our bodies.
Most closely linked to the subtopic Chemical Equilibria, the Chemistry of Aqueous Solutions deals with ionic equilibria and the behaviour of dissolved substances in water. Covering subtopics like Acid-base Equilibria and Solubility Equilibria, you will explore the dissociation of acids, bases, and salts and gain a deeper understanding of concepts like the buffer system and precipitation effect.
Some of the learning outcomes include:
- Recognising acid-base constants: understand and apply terms like pH; Ka; pKa; Kb; pKb; Kw in calculations
- Analysing solubility trends: discuss how factors like pH and common ions affect the solubility of ionic salts in aqueous solutions
Organic Chemistry
Most of the products we use today rely on knowledge gained from studying organic chemistry. Whenever you pop an aspirin or take your food to-go in a bag, remember where it came from!
Broadly speaking, Organic Chemistry is fundamentally intertwined with the Core Idea, Transformation, albeit taking a more in-depth look at carbon-based compound reactions. From wearing polyester clothing to using an alcohol-based hand sanitiser, you’ve already had plenty of exposure to products of organic chemistry.
Some of the learning outcomes include:
- Classifying organic compounds: identify between members of various homologous series (e.g. alcohols, amino acids, carboxylic acids, alkanes, halogenoalkanes, etc.)
- Illustrating electron movement: use the curly arrow notations to track electrons in reactions
- Explaining reaction mechanisms: analyse the factors that affect their rate and feasibility, such as the type of reaction (e.g., nucleophilic addition or oxidation)
Electrochemistry
One of the most common examples of electrochemistry is in batteries and rechargeable cells, where redox reactions and electrode potential are constantly at work.
Similar to Organic Chemistry, Electrochemistry is most closely aligned with the Core Idea of transformation, specifically, with the redox processes and electrolysis, which involve the transfer of electrons.
Some of the learning outcomes include:
- Defining the electrochemical terms: explain standard electrode (redox) potential and standard cell potential
- Performing electrolysis calculations: determine the quantity of charge and mass/volume of substance liberated during electrolysis
- Applying the ΔG⦵ = –nFE⦵ relationship: this also includes the calculation of E⦵ for combined half reactions
An Introduction to the Chemistry of Transition Elements
Due to their unique properties, transition elements have found a home in almost every industry, from being catalysts in industrial plants to being used in pigments and dyes.
This last extension topic is closely related to the Core Idea of Structure and Properties, as it focuses on the chemical properties of the first transition elements.
Some of the learning outcomes include:
- Defining transition elements: explain in terms of d-block elements forming one or more stable ions with partially filled d subshells
- Exploring redox systems: use Fe3+/Fe2+, MnO4–/Mn2+ and Cr2O72–/Cr3+ as examples of variable oxidation states
Free H2 Chemistry Crash Course Notes—The Right Way
Free H2 chem notes can be risky, but Zenith recognises that well-designed, syllabus-aligned notes can still play a valuable role, especially when created by experienced educators.
That’s why we’re offering free H2 Chemistry notes for our FREE June Crash Course, curated by our tutors for H2 Chemistry topics that align with the latest 2025 syllabus. These aren’t AI summaries or recycled school handouts—they’re designed to help you revise with clarity and purpose.
What’s inside:
- ✅ Concise topic summaries for quick review
- ✅ Key equations and definitions you must know
- ✅ Sample questions to test your understanding
- ✅ Tips on common pitfalls in each topic
These free notes are a great way to get a head start, especially if you're just beginning your H2 Chem journey or need a refresher before diving into deeper practice. Use them correctly: supplements to active learning, not as shortcuts.

📥 Download your free H2 Chemistry crash course notes from Zenith today and take the first step towards smarter, more structured revision.
What is the exam structure of H2 Chemistry? Paper 1-4 Explained
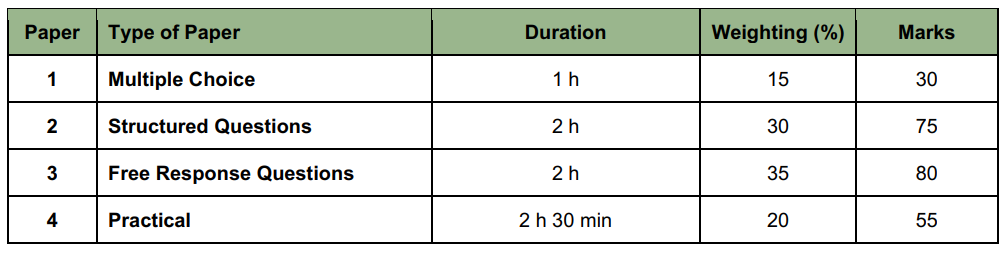
The H2 Chemistry exam is divided into four paper types. In this section of the article, we will discuss the exam structures of each paper, their assessment objectives, and some tips on what to focus on.
Paper 1 (30 marks)
Paper 1 has 30 compulsory questions, with each correct answer awarding you with 1 mark. You will have 1 hour to complete this exam, meaning that you can only spend an average of 2 minutes on each question.
Paper 2 (75 marks)
Paper 2 has a series of compulsory, structured questions, and mainly tests your ability to think critically within the scope of the syllabus. You will have 2 hours to finish this paper.
Paper 3 (80 marks)
Paper 3 is divided into two sections:
- Section A (55-60 marks): There will be 3-4 compulsory questions, each worth 15-20 marks.
- Section B (20-25 marks): There will be 2 questions, and students can choose to answer either one.
As the structure for Paper 3 has been updated in 2025, more precise details on the mark distribution will be revealed after the A-Level exams in 2026.
Paper 4 (55 marks)
Paper 4 is a practical exam, assessing the following four experimental skill areas:
- Planning
- Manipulation, measurement and observation
- Presentation of data and observations
- Analysis, conclusions and evaluation
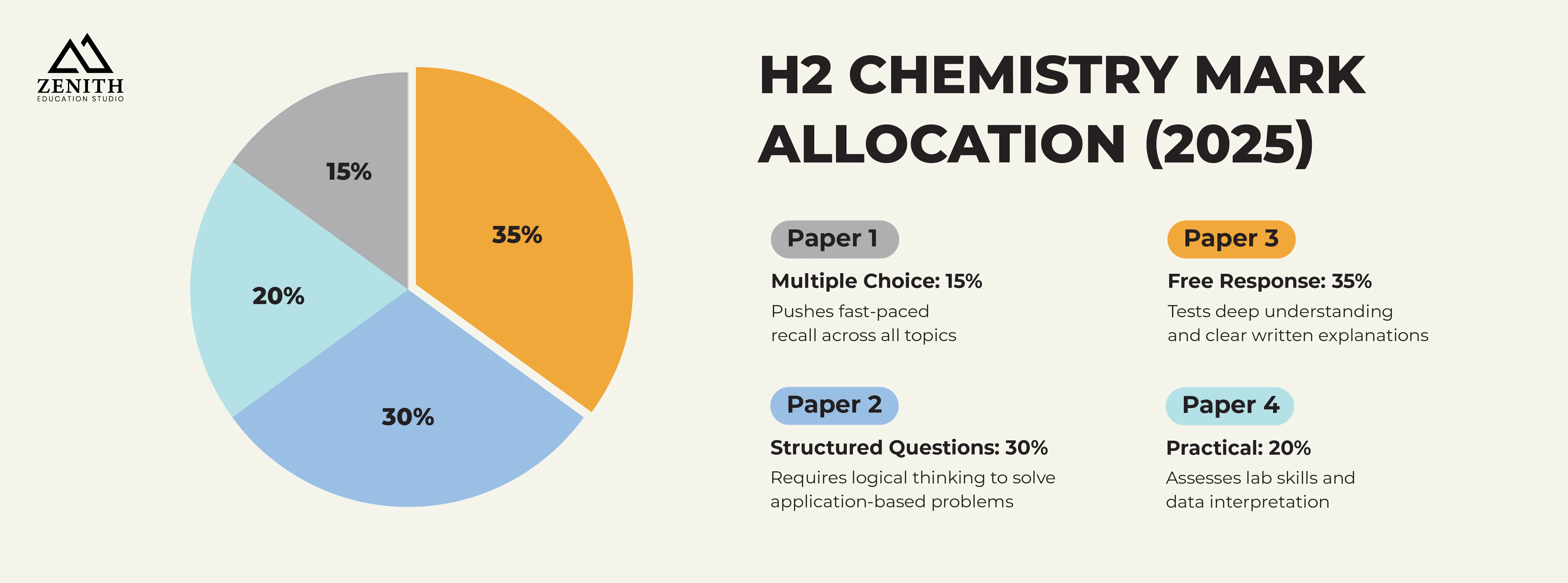
What is the mark allocation for H2 Chemistry?
The Free Response Questions in Paper 3 have the highest weighting, at 35%, which is why we recommend spending the most time studying for them.
The structured questions, with a 30% weighting, come in second place, followed by the Practical Assessment, with a 20% weighting.
Last but not least is Paper 1, the Multiple-Choice Questions, which have the smallest weighting of 15%.
It’s important to note that Papers 2 and 3 often include application-based questions, meaning that you will need to test your critical thinking skills while working under a time crunch.
Tips and Insights: Theme-by-Theme Guidance for H2 Chemistry
H2 Chemistry is structured into core themes that build upon each other progressively. At Zenith, Singapore's leading JC Chemistry tuition provider, we believe that understanding how to approach each theme strategically makes a real difference in mastering the syllabus. Here’s how you can tackle each theme more effectively:
Theme 1 (Matter) Tips: Adopt a Relearning Mindset
In this theme, be prepared to unlearn and relearn. JC Chemistry often challenges what you previously believed to be true at O Levels, like atoms having only eight valence electrons. You’ll now encounter concepts like subshells, orbitals, and electron configurations that go far beyond what you’ve seen before. Success starts with revisiting foundational knowledge and expanding your mental model.
- Be open to unlearning simplified O-Level concepts and embracing deeper A-Level theories.
- Revisit atomic structure focusing on orbitals, subshells, and expanded electron configurations.
- Understand that previous knowledge isn’t discarded, but expanded upon.
- Develop a habit of regularly reviewing and reassessing foundational core ideas.
_V1.webp)
Theme 2 (Structure and Properties) Tips: Look for Trends
Here, patterns are everything. You need to recognise how atomic structure influences bonding, and how bonding, in turn, affects properties like boiling point or reactivity. Don’t study topics in isolation—questions often combine concepts across chapters. Understanding trends like ionisation energies or bond strengths will help you interpret complex, cross-topic questions more effectively.
- Learn how structure affects physical and chemical properties.
- Focus on trends in bond strength, ionisation energy, and periodic patterns.
- Recognise that bonding concepts often appear with other topics (e.g., Organic Chemistry).
- Train yourself to interpret data and molecular behaviour across different contexts.
_V1.webp)
Theme 3 (Transformation) Tips: Master Calculations with Context
This theme focuses heavily on calculations, particularly around rates of reaction and chemical equilibria. But it’s not just about plugging in numbers. You must understand why a reaction happens and how fast it proceeds. Concepts like mole ratios, rate constants, and energy changes are tied closely together. Always link your numerical answers back to the feasibility and efficiency of the reaction.
- Build strong calculation skills around the mole concept, kinetics, equilibria, and thermodynamics.
- Understand the “why” behind formulas—feasibility, yield, and reaction rate matter.
- Know how to apply equilibrium expressions (Kc) and rate equations.
- Link concepts across chapters to tackle complex, multi-part calculation questions.
_V1.webp)
Theme 4 (Extension Topics) Tips: Connect the Dots
Extension topics like Organic Chemistry, Transition Elements, and Electrochemistry aren’t standalone—they draw deeply from earlier themes. For example, Organic Chemistry relies heavily on bonding concepts, while Transition Elements require a solid grasp of electron configurations. In Paper 3, your visualisation skills will be tested through structural elucidation and reaction mechanism questions, often demanding detailed sketches and logical deductions.
- Make connections between advanced topics and earlier themes (e.g., Organic Chemistry ↔ Bonding).
- Hone your ability to visualise molecules and reaction pathways.
- Practice structural elucidation using data and reaction outcomes.
- Be alert to visual cues like colour changes during titrations and practicals (especially for Paper 4).
_V1.webp)
Final decision-making guide
Now that you know what is in store for you if you take A-Level H2 Chemistry, you are in the best position to make the final decision.
However, if you’re still on the fence about whether to take chemistry or not, here’s a quick checklist to guide you on your choice:
If you answered ‘yes’ to 3 or more of these questions, then you might be ready to take on the challenge of H2 Chemistry. Hopefully, with this newfound knowledge, you can apply to your dream university with total confidence. Happy hunting!

References
1http://www.nus.edu.sg/oam/docs/default-source/admissions/h1-h2-sdp.pdf
2https://www3.ntu.edu.sg/oad2/website_files/brochure/emsr_ALevel.pdf
3https://www.sutd.edu.sg/admissions/undergraduate/singapore-cambridge-gce-a-level/criteria-for-admission
4https://admissions.smu.edu.sg/admissions-requirements/singapore-cambridge-gce-levels
5https://www.forbes.com/sites/rachelwells/2025/03/10/11-jobs-ai-could-replace-in-2025-and-15-jobs-that-are-safe


-min.webp)


.webp)
